At present, more than 99% of H2O2 is produced through high energy consumption and polluting anthraquinone oxidation pathways. In recent years, the method of reducing oxygen (2e-ORR) through a two-electron pathway to produce H2O2 has attracted attention. However, the existing catalysts in this approach are either expensive (noble metal catalysts) or only suitable for alkaline conditions. Therefore, there is still a need to develop 2e-ORR non-noble metal catalysts with high activity and high selectivity under acidic conditions.
Liquid-phase exfoliated molybdenum telluride (MoTe2) nanosheets can be used as high-efficiency materials. 2e-ORR catalyst has high activity in acidic electrolytes, which is the key to high selectivity: the jagged edges of MoTe2 nanosheets have a strong adsorption effect on the reaction intermediate HOO*, but the adsorption of O* is weak.
Preparation
MoTe2 nanosheets were prepared from top to bottom by liquid-phase exfoliation method. XRD and Raman results show that the MoTe2 nanosheets obtained by exfoliation belong to the hexagonal 2H phase. SEM and TEM pictures prove the successful peeling of MoTe2 nanosheets. TEM statistics show that the size of the nanosheets is between 50-350nm and the average size is 143nm. High-resolution spherical aberration electron microscopy observed the unique honeycomb atomic arrangement pattern and exposed zigzag edges of the 2H-phase MoTe2 nanosheets.
Catalytic performance
In an oxygen-saturated 0.5 M H2SO4 electrolyte, after combining MoTe2 nanosheets and graphene nanosheets, they exhibit a relatively positive bias potential (0.56 V relative to RHE) and excellent H2O2 selectivity (up to 93%) ), its mass activity (27) g-1at 0.4 V) is also second only to the best Pt-Hg and Pd-Hg alloys in the literature, and higher than Au-based alloys and N-doped carbon materials.
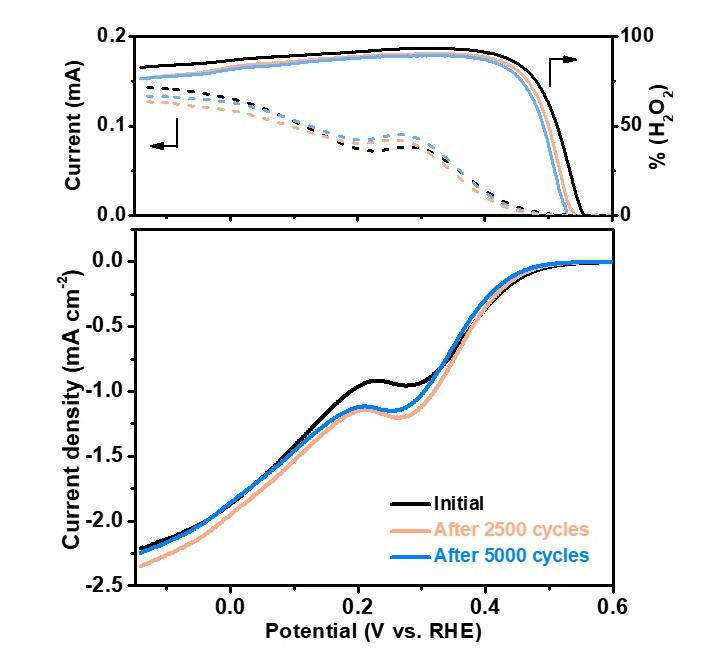
In addition, accelerated aging test experiments showed that after 5000 cycles, the activity and selectivity of MoTe2 nanosheets did not significantly decrease; after overnight aging, the activity only slightly decays, but there is no obvious selective decay. Experiments prove that MoTe2 nanosheets have good catalytic stability.
Principles of Catalysis
Theoretical calculations show that the proper adsorption of the zigzag edge of the MoTe2 nanosheets on the reaction intermediate HOO* ensures excellent 2e-ORR catalytic activity, while the weaker adsorption energy of O* is not conducive to the deep reduction of O*, thus Ensure high selectivity to H2O2. The two-dimensional heat map derived from the adsorption energy data of various materials on key reaction intermediates shows that PtHg4 has the best activity and selectivity, followed by MoTe2 nanosheets, while MoS2, MoSe2 nanosheets and Pt/C, etc. long distance. 2e-ORR is a highly active and selective region.
The above results show that MoTe2 nanosheets with jagged edges exhibit high activity, high selectivity and good stability in 2e-ORR, which provides new ideas and new options for the development of 2e-ORR acid electrolyte non-precious metal catalysts.


