Production Methods of Graphene
The production of graphene mainly adopts a variety of different preparation methods, each of which has its own characteristics and scope of application and the following are some of the main preparation techniques:
- Chemical vapor deposition (CVD):
– Enhanced Plasma Chemical Vapor Deposition (PE-CVD): this is a commonly used method for the preparation of monolayer graphene. A plasma is formed by heating a mixture of carbon-containing gases (e.g., methane, ethylene). Under certain temperature and pressure conditions, carbon atoms are deposited and ordered on a pretreated metal (e.g., copper, nickel) substrate to form a monolayer carbon structure (i.e., graphene.) During the CVD process, the rationing of gases and liquids is precisely controlled using a mass flow meter and controller to ensure the quality and thickness of the graphene film.
– Conventional CVD method: The substrate metal foil is placed in a furnace and first passed through hydrogen and argon or nitrogen as protective gases, heated to about 1000°C and held for some time to stabilize the temperature. Then change to pass into the carbon source gas (such as methane), in the high-temperature carbon atoms deposited on the substrate to generate graphene. Upon completion of the reaction, the power supply is cut off, the carbon source gas is turned off, the protective gas is re-introduced to expel the residual carbon source gas, and it is cooled to room temperature in a protective atmosphere. Finally, the graphene film is exfoliated from the metal substrate.
- Mechanical Peeling Method:
Tape Exfoliation Method: This method was initially prepared by Geim’s research group by manually exfoliating the graphite using 3M tape to prepare graphene. Although this method yields high-quality, defect-free graphene, the yield is extremely low, and the resulting graphene is small in size, making it unsuitable for industrial production.
- Reduction method:
– Graphene oxide reduction method: firstly, natural graphite is oxidized by strong oxidizing agents such as sulfuric acid and nitric acid to form graphite oxide (GO). Then, after washing and low-temperature drying, the graphite oxide powder is physically exfoliated (e.g., ultrasonic treatment) or expanded at high temperatures to obtain graphene oxide. Finally, graphene oxide is reduced to graphene using a reducing agent (e.g., hydrazine hydrate, hydrogen, etc.).
- SiC epitaxial growth method:
– Under ultra-high vacuum and high-temperature environments, silicon atoms sublimate to leave the SiC material, and the remaining carbon atoms form graphene through self-assembly so that graphene is directly grown on the SiC substrate. This method can obtain large-area, high-purity graphene.
- Other methods:
Solvent stripping, solvent thermal, high-temperature reduction, light reduction, epitaxial crystal growth, microwave, electric arc, electrochemical, etc., are also complementary means of graphene preparation, but they may be used in fewer applications or for specific occasions compared to the mainstream methods mentioned above.
For large-scale production of graphene, chemical vapor deposition (especially enhanced plasma CVD) has become an important route for commercial production due to its ability to prepare large-area, homogeneous graphene films with excellent properties and some potential for scale-up. However, the specific preparation process may need to be adjusted and optimized according to the actual needs, cost-effectiveness, and special requirements of the target application areas.
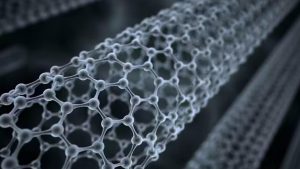
What is graphene?
Graphene is a two-dimensional carbon nanomaterial consisting of a single layer of carbon atoms in sp2 hybridized orbitals forming a hexagonal honeycomb lattice. It is an allotrope of the element carbon with a unique structure and excellent physical and chemical properties.
The following are the main features of graphene and its applications:
Characteristics of Graphene:
- Single-layer structure: Graphene consists of only one atomic layer thickness of carbon atoms, with a thickness of only 0.335 nanometers, making it the thinnest known two-dimensional material.
- High Strength: Graphene has extremely high tensile strength and is considered to be one of the strongest materials known. Its covalent bond structure gives it excellent mechanical stability.
- High conductivity: Graphene’s electrons can move at high speeds without scattering and have extremely high conductivity and carrier mobility, which gives it a significant advantage in electronics applications.
- High Thermal Conductivity: Graphene’s extremely high thermal conductivity far exceeds that of most conventional materials, including copper metal, making it excellent for thermal management applications.
- High Transparency: Although graphene is a dense arrangement of carbon atoms, it has a high transmittance of visible light, making it potentially useful in optoelectronic devices.
- Large Specific Surface Area: Due to its two-dimensional structure, graphene has an extremely high specific surface area, which is favorable for adsorption, catalytic reactions, and surface chemical processes.
- Tunability: Graphene’s properties can be tuned to meet the needs of specific applications through chemical modification (e.g., doping, oxidation, reduction) or compounding with other materials.
Applications of Graphene:
- Electronics and semiconductor technology: Graphene can be used to manufacture high-speed electronic devices, flexible electronic devices (e.g., wearable devices, folding screens), transparent conductive films (e.g., touchscreens, OLED display panels), as well as new types of transistors and sensors, which is expected to promote the progress of electronics and semiconductor technology.
- Energy field: With its excellent electrochemical properties and conductivity, graphene is used as electrode material for high-performance batteries (e.g., lithium-ion batteries and supercapacitors) to improve energy storage efficiency and charging speed. In addition, it has potential applications in solar cells (photovoltaic materials) and thermoelectric converters.
- Materials Science and Nanotechnology: Graphene has been used to develop lightweight and high-strength composites, such as reinforced plastics and metal matrix composites, due to its high strength, toughness, and good thermal stability. Meanwhile, it also shows great potential in nanosensors, nanoelectronic devices, and nanocomposites for thermal management and protective coatings.
- Biomedical applications: Graphene’s biocompatibility and bio-interactivity make it suitable for use in biosensors, drug carriers, tissue-engineered scaffolds, and medical devices for diagnostic and therapeutic purposes. Its unique surface properties facilitate precise drug delivery and cellular interactions, while in bioassay platforms, graphene can be used as a sensitive element for the detection of trace biomarkers.
- Other applications: Graphene may also be used in desalination, hydrogen storage materials, aerospace composites, photographic elements, and smart clothing combined with textiles (e.g., high-performance textiles with far-infrared functionality, antimicrobial, and breathable properties).
Luoyang Tongrun Nano Technology Co. Ltd. (TRUNNANO) is a trusted global chemical material supplier & manufacturer with over 12-year-experience in providing super high-quality chemicals and Nanomaterials, including boride powder, nitride powder, graphite powder, sulfide powder, 3D printing powder, etc.
If you are looking for high-quality graphene powder, please feel free to contact us and send an inquiry. (brad@ihpa.net)


